Museum Object Number: 40-13-196
AD 1519, central Panama: the conquistadors were angry. They had promised the Spanish court a mass of gold in return for investing in their risky transatlantic voyage. But having failed to find the legendary gold mines of the region, they had resorted to looting the treasuries of the local chiefs and the grave goods of native cemeteries. Then came the shock. As the soldiers began to melt down the heaps of ornaments they had gathered up, they discovered that scarcely anything was made of pure gold. Rather, the metal was debased with large amounts of copper.
The Spaniards’ melting pot contained a mix of gold and copper in roughly equal amounts: an alloy we now call tumbaga. They would not have been deceived by their plunder the alloy has a strong reddish tinge except that the native metal-smiths had artfully treated each object so that it had a micro-thin surface layer of near pure gold that shone with a spurious brilliance.
Research in recent decades has established how this deception was achieved. The tumbaga was first heated so that it became encrusted with a black scale of copper oxide. Pickling in an acidic plant juice then dissolved away the scale and preferentially leached out the copper in the underlying surface. What was left behind was a paper-thin layer of spongy gold. Rubbing with a slurry of fine sand or crocodile dung compressed that layer into a golden coating that completely belied the baser metal mix beneath. This technology (which we call depletion gilding) is thought to have been developed among the cultures of ancient Peru as early as 400 BC and to have been in use throughout Mesoamerica at least a millennium before the conquistadors arrived, In the course of preparations for a Museum exhibit of 1983 featuring treasures from the burials of Sitio Conte, Panama, I was impressed by how skillfully the early metalsmiths exploited depletion gilding. The golden luster of the various chest plaques and filigree jewelry that once adorned the deceased chief in Burial II gave a sense of how impressive the gold-bedecked body must have looked during the actual funeral some time around An Boo. This particular group of grave furnishings had withstood the ravages of nature far better than anyone might have expected.
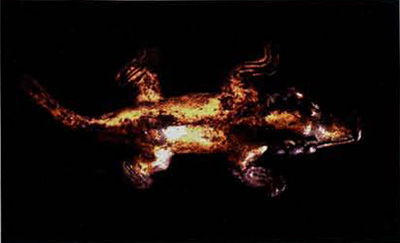
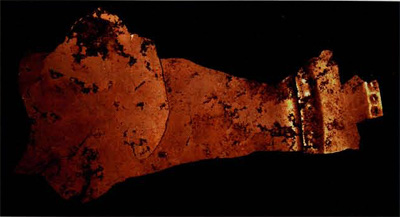
The gold furnishings from two other Sitio Conte graves, however, prompted me to probe more fully into the complexity of the tumbaga alloy. In Burials 18 and 25, there were perfectly intact depletion-gilded items alongside highly corroded ones (Figs. I and 2). Initially, I assumed that localized variations in the burial environment water seepage being the most likely would be enough to explain the difference in degree of preservation. Now, after a technical examination of the pieces, I am inclined to believe the answer is a more subtle one, involving something which, initially at least, occurs at a microscopic level, becoming visible to the eye only when the corrosive process is well advanced.
The gold-bedecked layer on a depletion-gildec artifact is prone to hairline cracking, particularly in more stressed regions such as a sharp edge produced by hammering or folding during manufacture. At the atomic level, once that layer is broached, the copper in the underlying tumbaga can oxidize to become the reddish brown mineral cuprite. If a substantial mass of cuprite corrosion does form, because it has a lower density than the surrounding metal it swells outward. The depletion layer then fractures at various points and is forced away from the tumbaga core (Fig. 3).
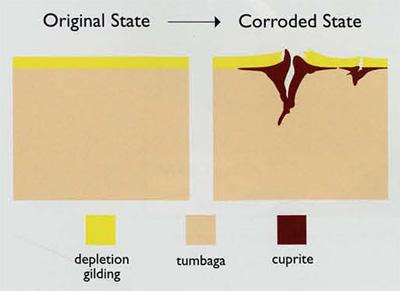
A fascinating aspect of this natural chemical process, however, is that tumbaga has something of a “self-healing” property. The amount of cuprite only builds up if the corrosion can tunnel deeply into the tumbaga. But given a sufficiently high proportion of gold in the copper alloy mix beneath the surface layer, gold atoms that have been set free by the corrosive process will migrate, coalesce, and seal over the sides of the minute channels where the attack is occurring. A depletion-gilded item made from a relatively gold-rich tumbaga might therefore suffer only minor surface pitting and retain most of its original luster, while a gold-poor piece within the same burial most likely would be heavily corroded.
What the different mixes of gold and copper in a tumbaga meant in terms of early Panamanian metallurgy we can only guess. Were the metal-smiths simply trying to save on gold by increasing the proportion of copper? or was a specific mix selected for a particular use? or might we discover that the relative levels tended to vary over time? At this stage we simply don’t know.
Stuart J. Fleming
Scientific Director, Museum Applied Science Center for Archaeology (MASCA)
Bibliography
Forty, A.J.
1979. “Corrosion Micromorphology of Noble Metal Alloys and Depletion Gilding”
Nature 282:597-98
Lechtman, H.N., A. Erlij, and E.J. Barry
1982. “New Perspectives on Moche Metallurgy: Techniques of Gilding Copper at Loma Negra, Northern Peru.”
American Antiquity 47:3-30.
